OralTox – For In Vitro Diagnostic Use
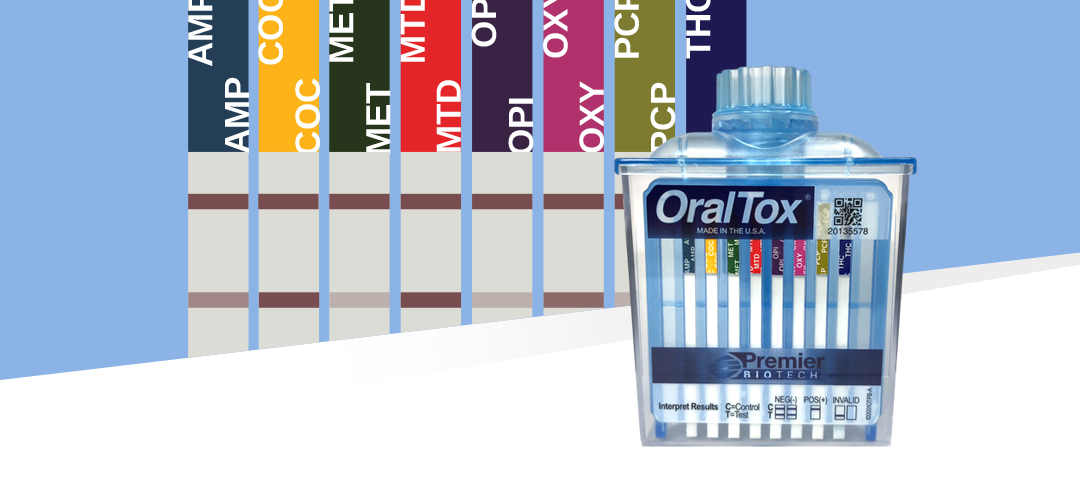
Introducing The First 8-Panel Rapid Oral Fluid Testing Device For In Vitro Diagnostic Use For Point-of-Care
We are thrilled to announce our patented rapid oral fluid device OralTox® has received clearance for two additional oral fluid assays, Oxycodone and Methadone. OralTox® is now available for in vitro diagnostic use for point-of-care for the following 8 drugs: Amphetamine, Cocaine, Marijuana, Methadone, Methamphetamine, Opiates, Oxycodone and Phencyclidine. This represents another landmark achievement that demonstrates our continued commitment to invest in the design, development and commercialization of near-donor solutions for the toxicology market.
By design, the OralTox format can scale to simultaneously detect up to 12 drugs, so this clearance marks the beginning of the next phase where more assays will be validated and submitted in an effort to fully utilize the capacity of the device. The use of oral fluid as a testing matrix offers numerous benefits over urine testing: the process is less invasive, the collection can be fully observed without privacy concerns, tests can be administered without the need for specialized collection facilities and, most significantly, is resistant to adulteration.
Todd Bailey, CEO, states, “Premier Biotech was founded with the goal of designing, developing and commercializing drug testing devices that represent the highest quality and most reliable results. Now with the clearance of Oxycodone and Methadone for OralTox, we are directly responding to the opioid crisis in the United States by enabling corporations and clinical groups alike to promptly screen and identify these illicit substances in a non-invasive manner.”
OralTox is now the only rapid oral fluid drug test for in vitro diagnostic use for point-of-care to include an assay for Oxycodone and is capable of being sent to a lab for confirmation. Our laboratory is uniquely positioned to offer LC-MS/MS confirmations of presumptive positive results as well as a comprehensive test panel with or without the OralTox front-end.
Our company has extensive experience working with corporate workplace and staffing organizations, clinical practices and government agencies to expedite their drug screening processes with innovative drug tests. After rigorous precision, safety, and efficacy review, OralTox enables further implementation of a rapid oral fluid drug test in these markets. Premier Biotech Labs holds both CAP (College of American Pathologists) Forensic accreditation and Clinical Laboratory Improvement Amendments (CLIA) certification, each of which requires the highest level of quality control standards and rigorous protocol adherence, including complete chain of custody and frequent inspections.
OralTox® is a registered trademark of Premier Biotech, Inc.
