
Certified Product And Sample Collection Training
Premier Biotech’s training program is intended to provide a means for a drug test administrator to develop an understanding of the Premier Bio-Cup product and sample collection process. Please read through all of the information within this training program. After completing the training program, you may test your knowledge with our online certification test. Once you have successfully passed the test with 100% accuracy, you will receive a personalized certificate of training in which you can print for your records.
Key Points to Remember From the Powerpoint (Video) Training You Just Watched:
- The cup being used is a 12 panel cup with AMP, BUP, BZO, COC, FENTANYL, METH, MTD, OPI, OXY, THC, TRAMADOL, K2 and Adulterant strip for pH, Specific Gravity, and Oxidants.
- Each strip has it’s own drug.
- Opiates and Oxycodone (along with other synthetic opioids like Buprenorphine, Fentanyl, and Tramadol) have their own specific test strips for optimized cross-reaction. A positive for one strip will not typically be the result of use of another. Ex. A prescription for Hydrocodone should not cause a positive on the Oxycodone (OXY) strip, while an Oxycodone prescription should not cause a positive on the Opiate (OPI) strip.
- The Temperature Strip located near bottom of cup should be read first to ensure sample is within 90-100 degree range.
- The Adulterant strip that delivers results for the sample being in the normal/abnormal range for pH, Specific Gravity, and Oxidants according to the color chart provided in the online portal. These should be read within 3 to 5 minutes after collection.
- An “abnormal” result does not mean a positive result or that the donor purposefully adulterated their specimen. This is something that should be documented in the “Comments” section of the product results portal and should be mentioned to the client to discuss why it may be “abnormal”. If “abnormal” results continue, try to switch up collection times and more closely monitor collection in order to get a valid sample in the “normal” range.

Product Overview
The Premier Bio-Cup is a preliminary screening test for the presence of drugs. The Premier Bio-Cup is a qualitative, immunoassay urine-based, rapidtest that incorporates collection of a urine specimen and the testing of multiple drugs simultaneously. For a quantitative result or to confirm a presumptive positive screen, a second, alternative chemical method should be performed on the same urine sample. Liquid Chromatography/Tandem Mass Spectrometry (LC-MS/MS) or Gas Chromatography/Mass Spectrometry (GC/MS) are the preferred confirmation methods.
Product Features And Benefits
- Test for multiple drugs simultaneously
- Customized panels with up to 16 drugs
- CLIA-Waived drug test strips available
- 60 minute test result stability
- Ability to test for specialty drugs: K2/Spice, Tramadol, Fentanyl, EtG, Alcohol, etc.
- Manufactured with automated technical manufacturing and semi-automated assembly
- Maintains test strip integrity
- Allows for customization
- Accuracy, sensitivity, specificity
- Proven accuracy, sensitivity and reliability
- Fast, accurate results in minutes
- Aggressive cut-off levels
- Easy to interpret results
- Cap closure indicator ensures sample will not leak
Storing And Packaging
- Premier Bio-Cup device should be stored at room temperature (15-30 degrees°C/ 59-86 degrees°F)
- Carefully inspect the foil pouch to ensure there are no tears, cuts, or damage to the packaging.
- Check the lot number and expiration date. Do NOT use if past the expiration date. The expiration date of the device is good through the last day of the month noted. Example: 2018-06 = June 30, 2018.
Procedure And Specimen Collection
- Have available for use a test results form/record template
- Require donor to present photo ID
- Ask donor to remove any unnecessary outer clothing and empty all pockets
- Secure the collection site, keeping all backpacks and purses out of the restroom
- Turn off water sources (sink faucets)
- Remove all substances that could be used to adulterate specimen (cleansers, disinfectants, soaps, etc.)
- Instruct donor to wash hands in your sight before donating a sample in order to remove any residue that may interfere with the specimen collection.
- Tear open the foil pouch and remove the cup.
- Hand the collection cup to the donor and request they provide the urine specimen.
- There is no minimum fill line on the Premier Bio-Cup. It is recommended however to ensure enough specimen to run the temperature strip. This allows the temperature strip to activate and ensures enough specimen is available if additional confirmation laboratory testing is required.
- After the donor has voided into the cup, replace lid and tighten it firmly to ensure urine will not leak. The Premier Bio-Cup has a cap closure indicator. When the indicated tabs are aligned, the cup is completely sealed.
- At this time, check the temperature strip to make sure the specimen is within normal range. The temperature of the specimen should be within 90-100 degrees Fahrenheit.
- Next, peel of the privacy label. The Premier Bio-Cup tests for one drug per strip. This makes the test easier to read as each strip contains its own control and test line.

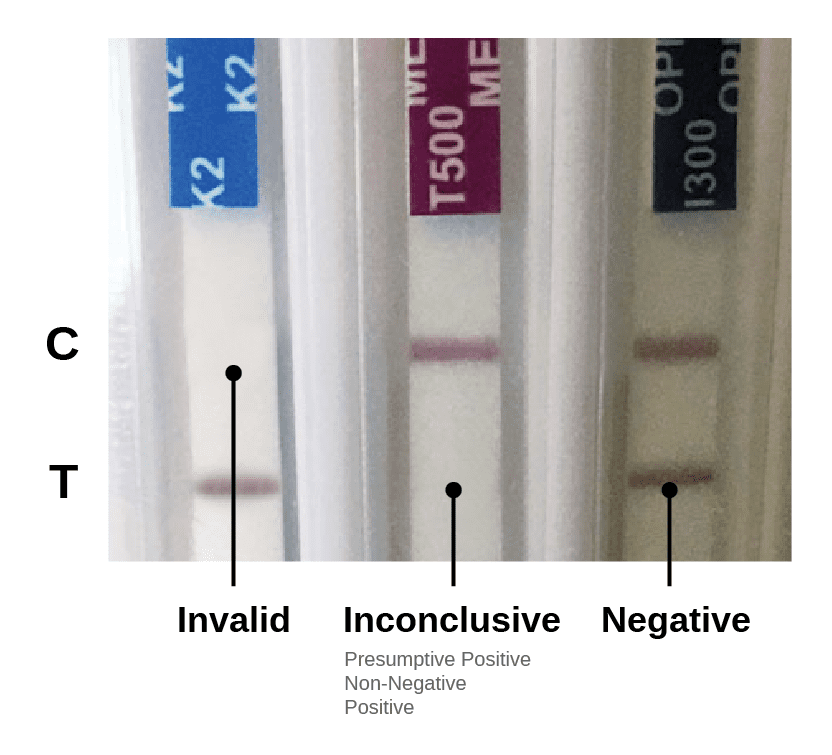
Results Interpretation
-
Make sure that the top control line is visible for all test strips, otherwise the test is invalid and the results must not be used.
-
Two lines appearing show a negative result.
-
Negative results may be read as soon as the top control line and bottom test line or lines appear.
-
Any test line regardless of the intensity, color or size, is a line and indicates a negative result. It is normal for line intensities to vary for different drug strips.
-
An Inconclusive result (also may be identified as positive, presumptive positive, or non-negative) is indicated by only the top control line present and the absence of a bottom test line.
-
Read Inconclusive results at 5 minutes.
- Results are stable for up to 60 minutes.
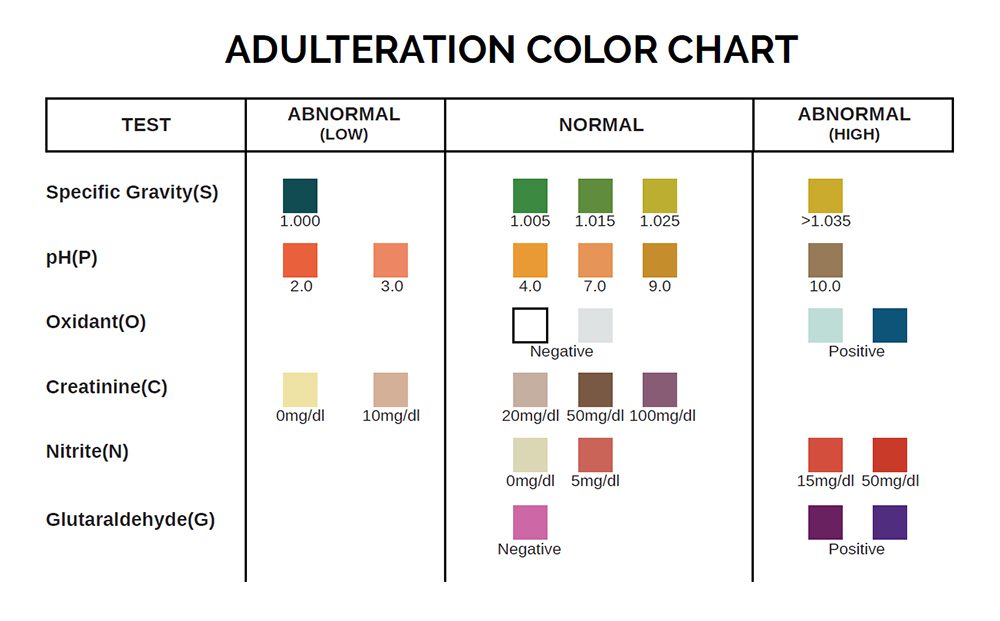
Specimen Validity Testing
The Premier Bio-Cup may come with specimen validity testing. The Premier Bio-Cup specimen validity test is based on the color response of chemical indicators in the presence of adulterants. Specimen validity tests help to determine the integrity of a urine sample. Read the specimen validity test results by visually comparing the color of the reagent pads to the corresponding color blocks on the specimen validity color chart at 3 to 5 minutes. Pad colors that change to a color NOT found listed in the normal range are to be read as abnormal.
The Chain of Custody (COC)
Step 1: The collector must identify the donor, either through prior knowledge and/or a State/Government issued photo ID.
Step 2:Have the donor initial and date the specimen ID/security label in the corresponding section as shown in red below. The agency information section (towards the top left) should be pre-printed. If this is not the case or any of the information is incorrect, please fill in or update appropriately.


Step 4:Ensure the specimen ID# on the COC matches the specimen ID# on thesecurity label used for the sample. At this time you can advise the donor to provide the specimen.
Step 5: Collector should affixthe specimen ID/security label over the device-first by centering the label over the top of the cap (as indicated on the label), than adhering the label flush down each side. This will create a security seal to ensure the specimen is not tampered with.
Step 6:The specimen is to be released to FedEx Express using the provided shipping label and supplies. FedEx Express provides the necessary courier.
Important: Every section of the Chain of Custody (COC) is very important and should be completed in its entiretyto ensure fast turnaround time and accurate reporting!

Reasons For Sample Rejection
Premier Biotech Labs is committed to providing clients with the most accurate results and therefore may reject samples for the following reasons. To ensure the highest quality standards and the integrity of each specimen, samples require proper collection, labeling and transportation.
Specimens submitted for testing must also be accompanied by the corresponding and completed Chain of Custody form as directed throughout this training. The following outlines reasons samples may be rejected once they have been received at the lab.

Test Your Knowledge By Taking The Certification Quiz
You have now completed the training. You may go back over any or all of the material above anytime. Once you feel confident with the material presented in the tutorial, you may proceed to the certification test by clicking the button below. After you receive 100%, a certificate will be generated on your screen and you may print from your computer for your records.





